Sorption Enhanced Reactions
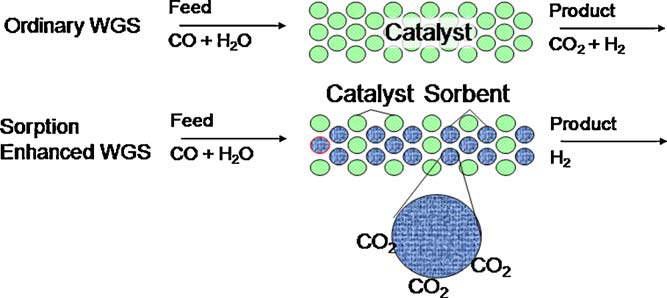
Sorption enhanced methane reforming and water gas shift reaction combines a high temperature CO2 sorbent and a high temperature shift catalyst to produce independent H2 and CO2 streams. Using novel lithiumorthosilicate (Li4SiO4) absorbent. Initial investigations showed CO2 absorption assisted water gas shift reaction at 600 C and simultaneous enhancement of the equilibrium conversion of a thermodynamically unfavorable reaction. Such an adsorbent increased the hydrogen yield two-fold above equilibrium concentrations. Preliminary studies show that the adsorption of CO2 in the sorbent is affected by a competitive increase in CO2 solubility at higher temperatures, affecting the reaction activity. The research is of prime interest in several gasification and reforming industries like Air Products, Praxair, Linde, etc. Use of such adsorbents during the reforming step can reduce the reaction temperature to 600 C (compared to 800-1000 C) while achieving high CH4 conversion to H2, prevent catalyst coking, reduce the need for excess steam, and reduce/eliminate downstream hydrogen purification steps.
Related publications:
- Investigations to Intensified Hydrogen Production Via Sorption Enhanced Water Gas Shift Reaction
Snehesh Shivananda Ail, Marco J. Castaldi, Anthony Vallace, Charles Coe, Michael Smith
The 27th North American Catalysis Society Meeting, New York, New York;2022
Modeling of a Non-Adiabatic Catalytic Reactor Backed By Experimental Data
The research done focuses on the reaction engineering of a catalytic reactor and a catalyst’s oxidation state change influencing its performance. Catalytic reactors are widely used in the industry for variety of applications ranging from emissions abatement to chemical synthesis. Designing a catalytic reactor requires understanding of kinetic performance of the catalyst as well as the mass transfer rates of reaction species from a bulk flow to the catalyst.
While the catalytic reactivity and selectivity derives primarily from the specific reactive sites on the surface for a given reaction, they are impacted by the rate of adsorption and desorption of reactants, products, and intermediates. The catalyst surface temperature would affect all of these at the surface transport and reaction steps. The combination of these steps forms apparent kinetic rate that can be derived from kinetic studies.
Operating under kinetic control the reaction rate and hence the reactor scaling is governed by intrinsic properties of the catalyst metal and its surface area coverage on the substrate material. Reactive species adsorb to the active surface site where they react requiring lower activation energy compared to the same reaction occurring in the bulk flow. The reaction rate follows Arrhenius law. For example, the power law rate expression for A + B → C + D would be:
r= -A*e(-Eact/RT)* CAaCBb
Where r is the kinetic rate, A is the pre-exponential factor, Eact is the activation energy, R is the ideal gas constant, T is temperature, CA and CB are molar concentrations of the reactive species and “a” and “b” are powers. From the rate expression it can be seen that it depends on three reactor parameters such as the catalyst properties, temperature and reactive species concentrations if “a” and “b” are not zero. An example of the kinetic rate expression as a function of temperature is shown in Figure 1 as the exponential curve. While Eact for the reaction depends on the catalyst type (i.e., catalyst metal used and in some cases its interaction with the catalyst substrate), the pre-exponential factor A depends on the number of the active sites available per unit mass of the catalyst as well as its accessibility to the reaction. The accessibility is mainly governed by the porosity, tortuosity, and the pore size of the substrate. The type of the substrate and its preparation of the substrate plays a significant role on these parameters. As for the active site amount it is mainly dependent on the catalyst metal loading % and its dispersion or average metal particles size on the substrate. Both, the physical parameters of the substrate as well as the metal dispersion can be affected by the catalyst particle geometry such as powder or larger scale spheres or pellets due to the different preparation methods of the substrate and the metal impregnation uniformity. Hence, it is important to validate the kinetic parameters for larger catalyst geometry after the lab scale kinetic study to properly scaleup the process.
Under bulk mass transfer-limited operation the rate of conversion is equal to the rate of diffusion to the surface of the catalyst. Assuming constant volume fluids (i.e., liquids) the following equation can be obtained for a packed bed reactor:
X = 1- e(-kc*ac*z/U)
where X is the conversion, kc the mass-transfer coefficient, ac the geometric surface area of the bed over the catalyst bed volume, and U the linear velocity. Hence, the mass transfer rate is a function of the geometric surface area (external surface of the catalyst) and the mass flux to the catalyst surface. The comparison can be extended to wash-coated substrates (i.e., high surface area supports like the sorbent/catalyst formula) via the Thiele modulus analysis, which is the ratio of the surface reaction rate to a rate of diffusion within porous solids.
Depending on the magnitude of these rates, either the kinetic or the mass transfer rates can be a rate limiting step that would determine how the reactor should be sized. Figure 1 compares the exponential kinetic rate trajectory that follows experimentally determined Arrhenius rate expression from Eq. (1) and the bulk to the catalyst mass transfer rate expression based on literature Sherwood number correlations. The kinetic rate starts at lower magnitude compared to the linear mass transfer rate trajectory, but quickly overtakes it at nominally 820K. This would be the point in a sizing model where the rate expression must be switched from mass transfer limited to kinetically limited rate if the reaction is endothermic or extra heat must be brought into the system to keep the reactor above 820K in the mass transfer limited regime. So, to properly size the gaseous packed-bed reactor the system of energy balance, molar balance and Ergun ODE equations were be solved in the model. Depending on which of the reaction rate type is rate limiting the rate terms in the energy and molar balance ODEs would change.
Overall, the experimental work combined the reactor modeling for larger scale of nominally 3 orders of magnitude and verifying the catalyst’s performance at the larger scale in comparison to the model prediction. In addition, a catalyst’s metal oxidation state change impact on the catalysts’ activity has been explored.
Catalytic Use of Ash in NOX Reduction
Due to challenges associated with source reduction, reuse, and recycling, waste to energy (WTE) remains a valuable technology in a portfolio of solutions for managing municipal solid waste (MSW). Specifically, the U.S. produces ~14 million MWh of electricity from ~30 million tons of MSW annually.[i] This amounts to only 12% of MSW produced annually with landfilling remaining the most prevalent disposal method due to economics and a limited understanding of thermal conversion.[ii] Increasing reliance on WTE hinges on reducing the technology’s cost. Specifically, there has been a recent acceleration in research of innovations that convert one of WTE’s liabilities into an asset: elimination of the disposal cost associated with MSW incineration (MSWI) ash by conversion into value-added products. Existing research in this area is limited, with most focused on the use of ash as an additive to building materials.[iii] My research aims to investigate MSWI ash in a less well-studied, but promising high-value application: as a catalyst in NOx reduction reactions. MSWI ash has demonstrated catalytic activity comparable to that of well-designed industrial catalyst[iv], but no concerted effort has been dedicated to understanding how its variable composition affects its catalytic activity or how stable it is under various reaction conditions.
Specifically, I will develop a kinetic understanding of NOx reduction over ash produced in MSW gasification and combustion such that a comparison can be made to those catalysts being employed in NOx mitigation currently. For reference, typical industrial catalysts employed in the selective catalytic reduction (SCR) of NOx exhibit ~90% conversion of NO when tested at 400 ºC with a reactor space velocity of 60,000 h-1.[v] Thus, benchmarks for measuring MSWI ash’s catalytic activity exist, and the initial focus of my project will be to synthesize ash with variable properties and make this comparison. Thereafter, the focus will shift to understanding performance as a function of time on stream. Typical industrial catalysts are susceptible to sulfur-poisoning and fouling by particulates. If activity comparable to industrial catalysts can be demonstrated, an understanding of the stability limitations is then required. In addition to consideration for the specific application of NOx emission mitigation, my project lies at the forefront of MSWI waste residual research, so my work is expanding the body of knowledge about these materials in general.
Furthermore, beyond the societal benefits related to MSW management, my research endeavors to reduce NOx emissions via a less costly catalyst, making treatment economically feasible in processes that presently preclude the use of expensive catalysts due to harsh environments and thus reducing emission of a potent greenhouse gas.
Related publications:
- Michaels, T.; et.al.: Waste-to-Energy Production. Energy Recovery Council’s Directory of Waste-to-Energy Facilities 2018, 5.
- Duren, R.; et.al.: California’s methane super-emitters. Nature 2019, 180.
- Joseph, A.; et.al.: The Use of Municipal Solid Waste Incineration Ash in Various Building Materials. Materials 2018, 1.
- Klinghoffer, N.; et.al.: Influence of char composition and inorganics on catalytic activity of char from biomass gasification. Fuel 2015, 37.
- Nakajima, F.; et.al.: The state-of-the-art technology of NOX control. Catalysis Today 1996, 109.
