Catalytic Use of Ash in NOX Reduction
Due to challenges associated with source reduction, reuse, and recycling, waste to energy (WTE) remains a valuable technology in a portfolio of solutions for managing municipal solid waste (MSW). Specifically, the U.S. produces ~14 million MWh of electricity from ~30 million tons of MSW annually.[i] This amounts to only 12% of MSW produced annually with landfilling remaining the most prevalent disposal method due to economics and a limited understanding of thermal conversion.[ii] Increasing reliance on WTE hinges on reducing the technology’s cost. Specifically, there has been a recent acceleration in research of innovations that convert one of WTE’s liabilities into an asset: elimination of the disposal cost associated with MSW incineration (MSWI) ash by conversion into value-added products. Existing research in this area is limited, with most focused on the use of ash as an additive to building materials.[iii] My research aims to investigate MSWI ash in a less well-studied, but promising high-value application: as a catalyst in NOx reduction reactions. MSWI ash has demonstrated catalytic activity comparable to that of well-designed industrial catalyst[iv], but no concerted effort has been dedicated to understanding how its variable composition affects its catalytic activity or how stable it is under various reaction conditions.
Specifically, I will develop a kinetic understanding of NOx reduction over ash produced in MSW gasification and combustion such that a comparison can be made to those catalysts being employed in NOx mitigation currently. For reference, typical industrial catalysts employed in the selective catalytic reduction (SCR) of NOx exhibit ~90% conversion of NO when tested at 400 ºC with a reactor space velocity of 60,000 h-1.[v] Thus, benchmarks for measuring MSWI ash’s catalytic activity exist, and the initial focus of my project will be to synthesize ash with variable properties and make this comparison. Thereafter, the focus will shift to understanding performance as a function of time on stream. Typical industrial catalysts are susceptible to sulfur-poisoning and fouling by particulates. If activity comparable to industrial catalysts can be demonstrated, an understanding of the stability limitations is then required. In addition to consideration for the specific application of NOx emission mitigation, my project lies at the forefront of MSWI waste residual research, so my work is expanding the body of knowledge about these materials in general.
Furthermore, beyond the societal benefits related to MSW management, my research endeavors to reduce NOx emissions via a less costly catalyst, making treatment economically feasible in processes that presently preclude the use of expensive catalysts due to harsh environments and thus reducing emission of a potent greenhouse gas.
Related publications:
- Michaels, T.; et.al.: Waste-to-Energy Production. Energy Recovery Council’s Directory of Waste-to-Energy Facilities 2018, 5.
- Duren, R.; et.al.: California’s methane super-emitters. Nature 2019, 180.
- Joseph, A.; et.al.: The Use of Municipal Solid Waste Incineration Ash in Various Building Materials. Materials 2018, 1.
- Klinghoffer, N.; et.al.: Influence of char composition and inorganics on catalytic activity of char from biomass gasification. Fuel 2015, 37.
- Nakajima, F.; et.al.: The state-of-the-art technology of NOX control. Catalysis Today 1996, 109.
Wet Waste Conversion
Nearly half of the 281 million tons per year (T/yr) of municipal solid waste (MSW) that is produced in the U.S. is sent to landfills. Waste-to-Energy (WtE) facilities are the only viable alternative to this that matches the scale of waste produced, producing electricity and reducing the waste volume. The declining cost of electricity due to increased natural gas and renewable electricity generation means the WtE facilities need to go beyond energy generation. MSW contains valuable materials that must be recovered and incorporated back into the economy.

This project focuses on the treatment of waste to energy ash with other waste streams like gypsum wallboard and spent Fluidized Catalytic Cracking catalyst (FCC) that can change the oxidation state of metals and convert them into more extractible forms. The testing involves the addition of non-MSW waste streams such as wallboard diverted from the construction and demolition streams and spent clay-based FCC catalyst wastes. Currently spent FCC catalysts are classified as non-hazardous and amount to nearly 400,000 tons annually which are currently sent to landfills. Gypsum waste is estimated at 13 million tons annually of waste produced annually with only 2% recycled. Therefore, if these materials can be profitably combined with the nearly 30 million tons of MSW annually processed in WtE facilities, a new avenue would open to increase the value of thermal processing facilities and recover the materials that currently are going directly to landfills.
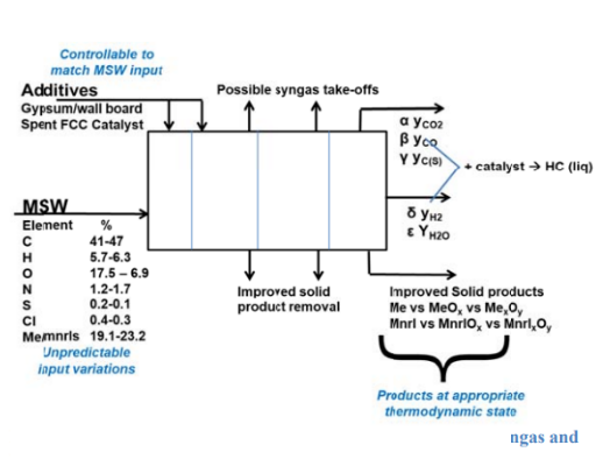
Therefore, the aim is to combine these waste streams with the MSW generated in the WtE plants and treat it thermally process it to recover valuable materials which are landfilled right now. The type of reaction that takes place is solid-solid between the ash and the additives at high temperatures forming metal oxides and different metal complexes. This leads to changes in the oxidation states of the metals and depending on the oxidation state, metal can be easy or difficult to extract. Hence, it is important to study the chemical reactions taking place between the MSW ash when mixed with additives like waste gypsum which is a part of the non-MSW waste stream at elevated temperatures in oxidative environments.
WtE facilities can be viewed as a thermal digester that homogenizes the highly variable incoming MSW into the lowest thermodynamic products of CO2, H2O, heat, and power. These products also have the least value. In the future, the homogenization process can be tuned to allow for the extraction of valuable and useful products and intermediates at higher thermodynamic states. The addition of gypsum into MSW ash has been shown to improve ash behaviour by converting metals like Al, Mn, Mg, and Fe into more extractible phases.
Related publications:
- Weckhuysen B. M., & Yu J., “Recent advances in zeolite chemistry and catalysis”, Chemical Society Reviews, 2015, 44(20), pp. 7022–7024. https://doi.org/10.1039/c5cs90100f
- Li Z., Qiu Z., Yang J., Ma B., Lu S., & Qin C. (2018). “Investigation of phosphate adsorption from an aqueous solution using spent fluid catalytic cracking catalyst containing lanthanum”, Frontiers of Environmental Science and Engineering, 2018, 12(6), pp. 1-11. https://doi.org/10.1007/s11783-018-1082-3
- Guetteche M. N., Zergua A., & Hannachi S. “Investigating the Local Granulated Blast Furnace Slag”, Open Journal of Civil Engineering, 2012, 02(01), pp. 10–15. https://doi.org/10.4236/ojce.2012.21002
- Castaldi M., “Impact of Additives to MSW for Pre-Combustion Enhancement of Syngas and Solid Residue Improvement”, DE-F Tech FOA-0001953 Technical Volume.
- Raj Goud Burra, K., Fernández, I., Castaldi, M. J., Goff, S., & Gupta, A. K.,” Effect of Gypsum Waste Inclusion on Gasification of Municipal Solid Waste”, J. Energy Resour. Technol. Feb 2023, 145(2): 021701.
